Imaging Directed Treatment for Biochemical Recurrence APCCC 2022 Presentation - Nicholas James
August 11, 2022
Biographies:
Professor Nicholas James, MBBS, FRCP, FRCR, Ph.D., Professor of Clinical Oncology at the Institute of Cancer Research at Royal Marsden Hospital, London
Almudena Zapatero: So now, we move to the debate we have in this moment, a topic, an interesting topic to debate. One from imaging directed treatment from biochemical relapse or PSA treatment directed biochemical relapse. For the first speaker, we have Dr. Nick James from Institute Cancer of Research in London. He's going to speak of imaging directed treatment for biochemical relapse pro.
Nicholas James: Right. Good afternoon. A great pleasure to be here once again for APCCC. So my disclosure is not terribly relevant to this talk, to be honest. So I'm going to focus on two separate things. The first is the question of when you start hormone therapy, if you've run out of local salvage options. So you've had post radiotherapy failure. You may have failed high field as well. The second is immediate versus deferred therapy with the respect to radiotherapy. And I'm going to kind of take a helicopter eye view, because we've already heard a lot of the detailed evidence as to what the principles are on which you might want to make decisions.
So does it matter when we start hormone therapy? So this is a question that keeps getting asked and asked and asked. And the first trials that asked this came out in favor of early hormone therapy, like this MRC trial, but it's worth pointing out that the late treatments in this trial was super late. 10% of the patients died of prostate cancer without getting any treatment at all. And they all had advanced disease at study entry. So treatment versus no treatment in some for advanced disease where you're not giving any other treatment, early treatments better. But appropriately enough, because we're in Switzerland, I've highlighted a SAKK trial, more solidly in the PSA era asking the same question. So again, elderly men immediate or deferred orchiectomy and orchiectomy deferred to symptomatic progression. So again, late salvage.
And in the PSA era, there's now no survival difference from early versus late. So it seems to be pretty good evidence that in the biochemical occurrence setting where you've got much, much less disease, it's got to be safe to defer treatment. And a slightly different take on the same thing, which is intermittent versus continuous hormone therapy. So the Intercontinental trial, absolutely not a smidge of difference between these two curves. So that the message would appear to be that you can safely defer or reduce treatment with hormone therapy in the PSA recurrent setting.
And it's particularly important to remember, because the next few slides are on this. There's probably no survival benefit to early versus late hormone therapy. With better monitoring as compared to those earlier trials, it's unlikely to have made it less safe. It's likely to have made it more safe. And the harms of overtreatment hormone therapy are well known. And so the question is, can we predict who's at highest risk and therefore might need the earliest treatment? And I think this is particularly relevant when we come to look at the data because there's a kind of a message that the higher your PSA, when you get your salvage treatment, the worse the outcomes. But I'm going to give you an alternative explanation for that, which is it's not the lateness of the treatment. It's the rate of the PSA rise that is driving those differences.
So again, it's worth looking at old data here and we shouldn't forget that, yeah, we've been studying this sort of question for a long time. So this is the very old now, but there's no reason to think it's changed, Pounds data. So on the left hand box, you can see that the higher the Gleason score, the bigger the risk of developing metastases. But nonetheless, even for the highest Gleason scores, you've still got a five year median time to metastasis after PSA failure.
If we look at the time to PSA recurrence, as you'd expect, the later your PSA goes up, the longer the time to metastasis. You've got a slower tempo of disease. And finally, if we look at the PSA doubling time and the time to metastasis again, you've got a very clear effect for fast versus slow. So I would suggest that these last two things are the thing that's driving the PSA effects that we see at time of salvage. It's that if you've got a fast rising PSA, you're likely to have a higher PSA. By the time you get salvage, you've got more aggressive disease. You're likely to have a worse outcome, and it's not the early versus late salvage that's the key here. It's the underlying biology of the disease that is driving the differences seen at PSA at induction of salvage radiotherapy.
And I'd just like to remind you, there's lots of harms associated with giving people too much hormone therapy. I've just chosen to highlight one here, but we've already seen the cardiovascular issues highlighted in the RTAG trial by Brandon Mahal and the effects of giving too much hormone therapy kick in really quite early. So this is [inaudible] data, 50 odd thousand men. These are real fractures. These are not radiological fractures like the [inaudible] trials, where there was a lot of imaging. These are fractures where people went, "Ouch" and had an x-ray and there was a break. And as you can see, once you get past four doses of sulodex or equivalent, you are up into... Yeah, quite a big risk of fracture. By the time you've got lifelong ADT, you've got a huge risk of fracture, more than double the risk in patients having no hormone therapy at all.
So the implications from starting ADT are that many men will die from other causes, just have shown you the metastasis free survival data. So we have to be very careful to not start it too early and give too much of it. Even if you develop metastasis, a lot of these men are still going to die from other causes, and I'll show you the oligomet recurrent versus de novo data in a second. And all men are guaranteed to be harmed by early use of ADT. And the harms are well known. I don't need to spell them out to this audience.
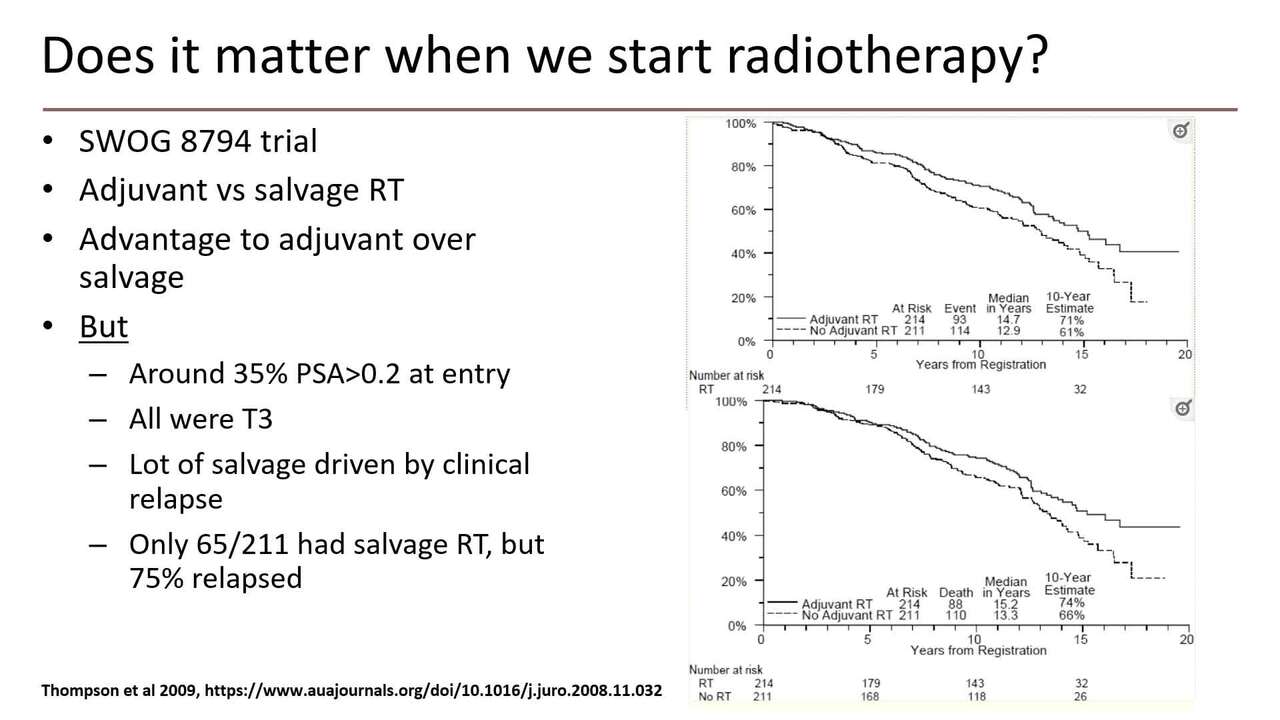
So moving on, does it matter when we start radiotherapy? So I'll go back to the earliest of these trials, SWOG 8794, 430 men, and there's a clear advantage to adjuvant over salvage radiotherapy in this trial, including a survival advantage. But a lot of these men had kind of already failed before they went in the trial and there was an underuse of salvage radiotherapy.
So I think this is a bit like the MRC immediate versus the first hormone therapy trial. This shows that the treatment works, but it doesn't tell you what happens if you give the same treatment later, because not enough men got the same treatment later to be sure. So we go to a later trial, this is Michelle Bolar, first author of the EORTC trial, adjuvant versus salvage with radiotherapy on relapse. Time to PSA failure is better, but in this, your PSA failure you'd expect to be better if you didn't get as much treatment up front. But if we play it forward into progression free survival, then find overall survival, there's really not a lot of suggestion that early versus late is any better. In fact, there's even possibly a suggestion of harm here. And then the final trial already been shown is the radicals trial.
So you've got key inclusion criteria, which are high risk features. And I just put some of these in. They're pretty high risk patients in the trial, T3, positive margins, lymph node involvement, high CAPRA score. So these are high risk men. And the striking thing about this. So the top bar here is biochemical progression free survival. So if your PSA goes up and you get salvage radiotherapy, that doesn't score on this, unlike the Ebola trial. And you can see you've got very, very good biochemical relapse free survival with only a third of the men, or just under getting salvage radiotherapy. So two thirds of the men get the same outcome, but with no treatments. And if you look at the bottom graph, freedom from non-protocol hormone therapy. So hormone therapy and associated with salvage radiotherapy doesn't score here. Again, you've got very, very high rates.
And I think the other thing that is to highlight here is that we had to change the outcome measure for radicals because there simply weren't enough death. So there's not even a hint of a survival difference with this with later radiotherapy, but there is difference in the toxicity, as again as already been highlighted. So does it matter when we start radiotherapy? With newer data, it's very hard to show benefit from adjuvant as opposed to delayed treatment. And you can debate how delayed is delayed of course. It suggests better imaging plus PSA monitor keeps patients safe and there's the side effect penalty from overtreatment.
So the final thing is around about what you're going to do with your radiotherapy. So if we treat on PSA alone, how likely are we to treat sites of occult disease? And I've highlighted a paper published earlier on this year, mapping sites of recurrent following adjuvant or salvage radiotherapy in prostate cancer patients. And the thing to note is that if you give a bigger field, PB is prostate beds, PB plus PLN is pelvic lymph node. Your pelvic lymph node relapse rate goes down. So radiotherapy works as we know. If you radiate the disease, you're more like to stop it coming back. But the thing that's striking here is the very high rate of metastatic recurrence.
And this is probably not going to be impacted by your use of pelvic radiotherapy I would argue. And just to highlight, these scans, the median PSA and the PET scan components of this is 1.4. So you have to hypothesize that you're metastasizing whilst your PSA is in the low single figures and that you can prevent it by giving early radiotherapy, which given that mostly metastatic disease doesn't appear until your PSA is 20, 30, 40, it seems intrinsically unlikely. It seems much more likely those mets were already there, just quietly growing because you never treated them.
And this is another figure from the same paper. The figure is actually the numbers are wrong. So there's 244 radiologically sites of failure. Now the ends there, just ignore the 0.5% or whatever. The ends are the numbers. So very few local failures. Local regional failures, predominantly in those who didn't get local regional treatment, but then a substantial number of metastatic failures. So the last few slides are around oligometastatic disease. So again, I'm not going to go too much into Sabre and stuff like that because that's in another session. But just to highlight that metachronous metastatic disease has a different prognosis to synchronous metastatic disease, two different studies. One's data from [inaudible]. The other is again US Registry data. So much, much better prognosis for oligo recurrent disease, as opposed to oligo presenting disease. And again, you're going to see these slides over and over.
I'm sure if you treat all the mets, you get a much better outcome than if you don't treat all the mets. And particularly if you go down into PET detected recurrents, Sabre works much better if you treat the stuff that you see and detect on PET, as opposed to the stuff detected on conventional imaging, just recapping the previous discussion a bit. And there may well... Although just talking to Pete [inaudible] just now at coffee, there may not be differences that you can predict on the underlying biology. So this is the published data. And so, it's probably the biology that drives your chance of getting met as much as the timing of when you give treatment.
So the conclusion I would have is that PSA tells you about the presence of disease, and the PSA kinetics for prognostic, but it doesn't tell you where the relapse is going to occur. Relapse patterns post radiotherapy show that almost all the relapses occur outside the radiotherapy field, not inside it. Deferred treatment, both with hormones and radiotherapy appears safe in terms of overall survival, which I would argue is ultimately the most important thing. And also very important, you've got evidence of potential harm from early treatment, both early hormones or early radiotherapy, because you're going to overtreat or miss the target. There's also very good evidence that radiotherapy works, which is reassuring given that it's what I do for a living. But if you don't irradiate all the disease, relapse is pretty much inevitable as the [inaudible] and Stomp trials show, for example. So I would argue, therefore coming back to my remit, the image directed policy ensures the best treatment decisions. It protects you from overtreatment, makes it more likely you're going to do the right treatment. Thank you very much.


